Abstract
Background: Acute myeloid leukemia is an aggressive malignancy with poor outcomes especially in patients 60 years of age or older. This thought to be in part from increased resistance to chemotherapy in AML cells arising in an older host. One of the nine recognized biological hallmarks of aging is a decline in mitochondrial quality. The effect of exploiting this age-related metabolic vulnerability in relapsed AML has not been previously established. CPI-613 is a first-in-class agent that inhibits pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, effectively impairing the TCA cycle. We have combined CPI-613 with high dose cytarabine and mitoxantrone in phase I and II clinical trials in over 100 patients with relapsed or refractory AML.
Methods: To determine the effect of the addition of CPI-613 in older patients, the phase I and II datasets were combined and outcomes by age were analyzed and compared to age related outcomes in a historical dataset of patients treated with a high dose cytarabine, mitoxantrone and L-asparaginase but without CPI-613. In both trials, patients were given CPI-613 as a 2-hour infusion on days 1 through 5. Cytarabine was dosed at 3,000 mg/m² (if younger than 60) or at 1,500 mg/m² (if 60 years of age or older), given every 12 hours for 5 doses, starting on day 3 following the CPI-613 infusion. The mitoxantrone was dosed at 6 mg/m² and is given once daily following the first, third and fifth cytarabine doses. At day 14, nadir marrow was evaluated, and patients with residual disease could be re-treated as above or with an abbreviated 3-day cycle. Responding patients were eligible to receive up to 2 abbreviated 3-day consolidation cycles. The historical cohort was treated identically without CPI-613, except L-Asparaginase was given at a dose of 6,000 units/m2 following the last dose of cytarabine.
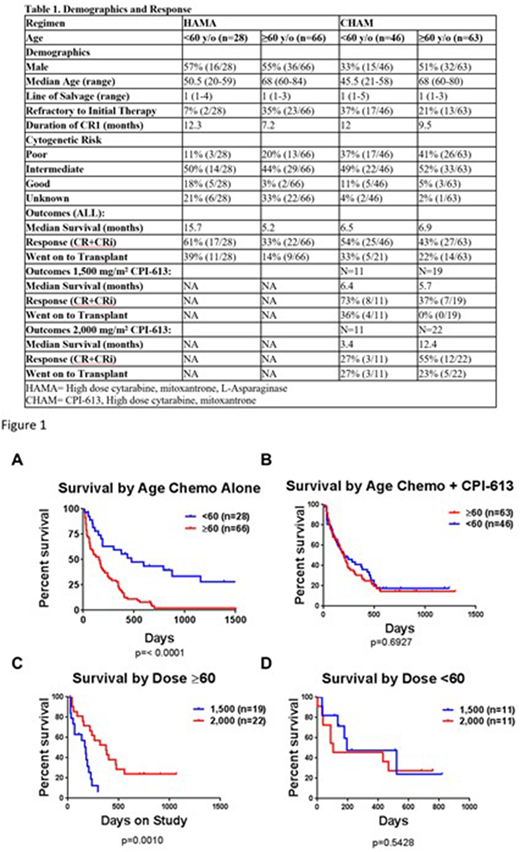
Results: Patient characteristics are summarized in Table 1. In the historical dataset younger patients had a highly significant increase in median overall survival when compared to patients ≥60 years of age (figure 1A). In contrast, older and younger patients treated with CPI-613 in addition to the chemotherapy had no significant difference in median survival (figure 1B). Additionally, when outcome by dose of CPI-613 was analyzed, older but not younger patients had a significant improvement in survival when given a dose of 2,000 mg/m2 compared to those given 1,500 mg/m2 (figure 1C+D). Patients 60 years of age or older had a response rate of 55% and a median overall survival of 12.4 months when treated with a dose of CPI-613 of 2,000 mg/m2.
Conclusions: Targeting mitochondrial metabolism exploits an age-related metabolic vulnerability in AML arising in older patients. These results have led to a randomized phase III trial of CPI-613 at 2,000 mg/m2 in combination with high dose cytarabine and mitoxantrone compared to high dose cytarabine and mitoxantrone alone in relapsed or refractory AML patients 60 years of age or older.
Pardee:Amgen: Speakers Bureau; Karyopharm: Research Funding; Celgene: Speakers Bureau; Rafael Pharmaceuticals: Employment; Novartis: Speakers Bureau. Ellis:Alexion: Speakers Bureau. Powell:Rafael Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.